
Milk is an example of an o/w emulsion, in which the fat phase or cream forms tiny droplets within the skim milk, or water phase. Simple emulsions are either oil suspended in an aqueous phase (o/w), or water suspended in oil (w/o). These examples represent emulsions, which are stable mixtures of tiny droplets of one immiscible fluid within another, made possible by chemicals called emulsifiers. The immiscibility of oil and water has inspired the proverb “Oil and water don’t mix” and other expressions that reflect the general incompatibility of two entities, such as “My coworker and I are like oil and water.” Yet within our homes are numerous examples of products in which oil and water do mix: mayonnaise, milk, salad dressings, hand lotion, and hair conditioner, to name but a few.

how emulsifiers are used in foods, nutraceuticals, personal and home care products, industrial lubricants, environmental technologies, biofuels, and other applications.how formulators choose which emulsifier to use for a particular emulsion.To learn more about separating an oil emulsion, check out our blog 6 Ways to Separate an Oil Emulsion.After reading this article, you will understand: When the film is broken, the water droplets coalesce to form bigger drops, which will settle to the bottom because water is heavier than oil. There are four things that producers use to break the emulsion: To treat and separate an emulsion, you need something to weaken the film surrounding the water droplets. The film must be broken in order for the water droplets to coalesce and fall out. If it is composed of droplets which show a tendency to separate, it is known as a “loose” or “unstable” emulsion.Įmulsifying agents create a film around water droplets and hold it in the oil. This middle emulsion layer will often remain as an emulsion indefinitely.Īn emulsion composed of extremely small droplets that shows no tendency to separate into water and oil is known as a “tight” or “stable” emulsion.
#WATER IN OIL CREAM EXAMPLE FREE#

The emulsifying agent may be naturally occurring elements like paraffin or chemicals used when drilling the well. This film is a layer of emulsifying agent, and it prohibits the droplets from bumping into one another so they can coalesce. Each of these tiny spheres is surrounded by a tough film. If you looked at the emulsion through a microscope, you would see a great number of tiny spheres of water mixed throughout the oil. It may have a fluffy appearance and feel, which is caused by gas bubbles trapped in the emulsion. In other words, the emulsion is thicker and will not flow as readily as oil or water. Generally, the viscosity of the emulsion is higher than the viscosity of the oil or water. For example, a dark green crude oil when emulsified will often appear a muddy brown color. In appearance, the emulsion resembles neither oil nor water. Water-In-Oil and Oil-In-Water Emulsion Examples Mixing is even more pronounced in pumping or gas lift wells. Upon reaching the surface, more violent action takes place as the fluids pass through a choke. While the emulsion travels up the tubing toward the surface, mixing and agitation continue and intensify as gas bubbles are released. When water and oil in a reservoir enter the well bore through the perforations in the casing, large pressure differences are created which violently mix them together. Rather, it is formed when oil and water are produced together with a great amount of agitation. This is called a “reverse emulsion.” How are Oil Emulsions Formed?Ī crude oil emulsion, also called a petroleum emulsion, does not normally exist downhole in a producing formation. Occasionally, an emulsion occurs that contains droplets of oil dispersed in water. In a crude oil emulsion, the quantity of water droplets is usually less than 10%.

It contains fine water droplets dispersed in oil.
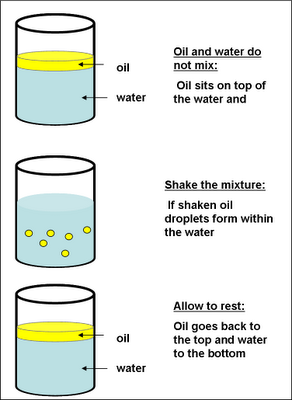
An oil emulsion is a mixture of oil, water, and an emulsifying agent.


 0 kommentar(er)
0 kommentar(er)
